|
Accelerating Commercialization of its Novel Pain Monitoring Solutions
RAMAT GAN, Israel, Sept. 22, 2020 /PRNewswire/ — Medasense Biometrics Ltd., developer of the NOL technology for pain-response monitoring, today announced that it has raised $18M in a series C round, from Sabadell Asabys venture capital firm (Asabys Partners, Spain), Israeli family offices and returning investors Baxter Ventures, Olive Tree Ventures and LGL Capital.
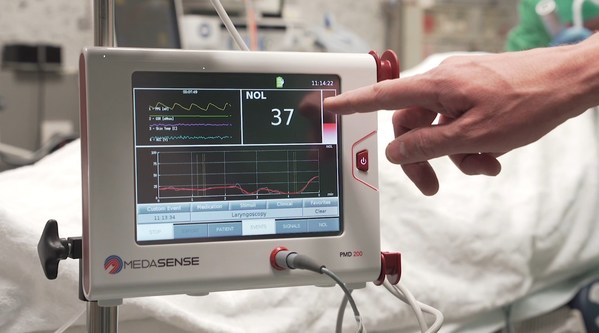
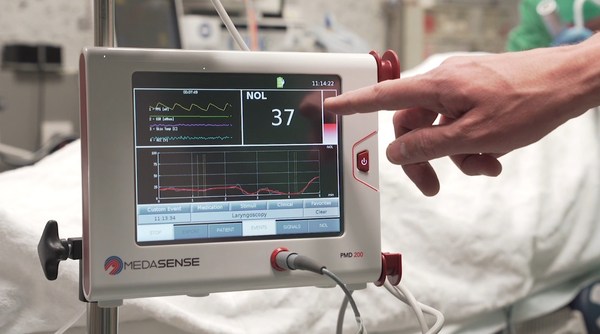
Medasense’s NOL technology for pain-response monitoring enables clinicians to personalize treatment: control pain, avoid overdose, and eliminate doubt. The technology is currently utilized in operating rooms and critical care settings, where patients are under anaesthesia and unable to communicate.
Considering the complex nature of pain, Medasense technology uses a unique multi-parametric sensor platform and advanced AI algorithms to convert complicated data into a patient’s “Signature of Pain.” The technology, which is currently utilized in operating rooms and critical care settings, where patients are under anaesthesia and unable to communicate, enables clinicians to personalize treatment: control pain, avoid overdose, and eliminate doubt. The company has also been active in the implementation of its technology on COVID-19 ventilated patients.
Studies have shown that NOL monitoring can potentially reduce hypotensive events and opioid consumption during surgery,[1] reduce postoperative pain experienced by patients in the post anaesthesia care unit,[2] and reduce cost of care. This addresses a widespread need. It is estimated that 50% of surgical patients suffer from moderate to severe postoperative pain and 12% suffer adverse events due to pain relief medication. These can result in extended hospitalization, additional healthcare costs, and a 50% increase in hospital readmissions.
Josep Ll. Sanfeliu, founding partner of Asabys Partners, stated: “Medasense is a model startup, having successfully introduced a disruptive medical device technology to the market. Its artificial intelligence solution is impacting clinical outcomes to benefit patients and to enable better use of healthcare resources.”
“Together with our trusted investors, who share our passion to improve pain management, we expect to make a significant contribution to enhancing pain care,” said Galit Zuckerman-Stark, Medasense founder and CEO. “This funding round will allow us to expand and further consolidate commercial deployment of NOL technology in Europe through our distribution agreement with Medtronic and to complete the process of obtaining FDA approval for commercialization in the U.S.”
About Medasense and NOL Technology
Medasense (www.medasense.com) offers a breakthrough technology that enables clinicians to personalize pain control and avoid overmedication. Medasense’s flagship product, the PMD-200™ with its NOL® index, is a unique platform that objectively monitors and quantifies the patient’s pain response by means of a proprietary non-invasive sensor platform and artificial intelligence.
The PMD-200 is used to optimize pain management in critical care and operating room settings, where patients are unable to communicate.
Clinical studies have demonstrated its impact on patient safety and outcomes, including opioid sparing, less postoperative pain experienced by patients in the post anaesthesia care unit, and reduced cost of care.
The PMD-200 is distributed in Europe exclusively by Medtronic, is cleared for marketing also in Canada, Latin America, Israel and Australia, and enables connectivity with Philips patient monitors.
Watch Medasense’s 1-minute video
1. Meijer, F., Martini, C., Broens, S., Boon, M., Niesters, M., Aarts, L., Olofsen, E., van Velzen, M., Dahan, A. (2019). Nociception-guided versus Standard Care during Remifentanil–Propofol Anesthesia: A Randomized Controlled Trial. Anesthesiology, 130(5), 745-755.
2. Meijer, F., Honing, M., Roor, T., Toet, S., Calis, P., Olofsen, E., Martini, C., van Velzen, M., Aarts, L., Niesters, M., Boon, M., Dahan, A. (2020). Reduced postoperative pain using Nociception Level-guided fentanyl dosing during sevoflurane anaesthesia: a randomised controlled trial. British Journal of Anaesthesia, In Press. DOI: https://doi.org/10.1016/j.bja.2020.07.057.
Photo – https://mma.prnasia.com/media2/1278423/medasense_nol_monitoring_surgical_settings.jpg?p=medium600
Logo – https://mma.prnasia.com/media2/1169999/Medasense_Logo.jpg?p=medium600
For further information please contact:
Mira Sofer, VP Biz Dev & Marketing, Medasense
[email protected]
Related Links :
Home page