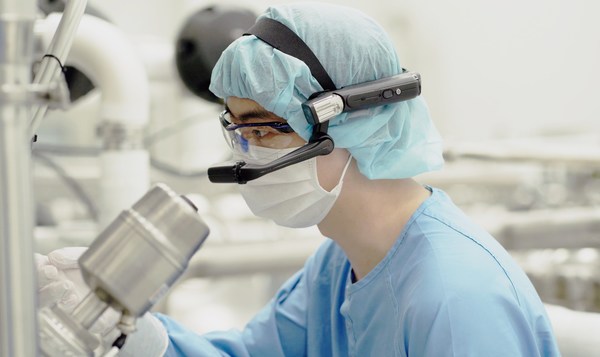
- Samsung Biologics’ Live Virtual Tour (LVT) used in one of the earliest FDA full virtual assessments
- Recently hosted EMA for 6 PAIs simultaneously using LVT
INCHEON, South Korea, June 23, 2021 /PRNewswire/ — Samsung Biologics, a global contract development and manufacturing organization (CMDO), celebrated the one-year mark of Live Virtual Tour (LVT), a real-time interactive platform to provide remote site inspections for both clients and regulatory agencies. The innovative digital solution was developed and implemented at Samsung Biologics a year ago in the wake of the pandemic and has since been successfully utilized in over 50 virtual regulatory and client audits and inspections and counting.
When many regulatory agencies and clients had postponed or completely discontinued GMP site inspections due to COVID-19 travel restrictions, Samsung Biologics proactively leveraged its process innovation to custom tailor a robust solution specifically to address the rigorous demands of a biopharmaceutical onsite inspection. Live Virtual Tour is an omni-device platform which allows third parties, such as the FDA and clients, to remotely observe and assess Samsung Biologics’ facilities, communicate in real-time, and review GMP documentation, all in high definition using a flexible and secure global cloud infrastructure.
Since the launch of LVT, the company has utilized the platform for many aspects of its business, including technical due diligence and regulatory inspections including one of FDA’s earliest virtual assessment for Emergency Use of Authorization (EUA) of COVID-19 treatments, and more recently, EMA’s virtual inspection ("EMA distant assessment") for six Pre-Approval Inspections (PAI) over the course of seven days, receiving no critical observations.
John Rim, CEO of Samsung Biologics, said of the inspections, "While we have had many successful regulatory inspections in the past, these recent approvals, are meaningful milestones for Samsung Biologics. We will continue to adopt new technologies and stay abreast of what regulatory authorities require to help our clients secure robust and safe delivery of treatments to all patients around the globe."
The FDA issued a new recommendation in April providing guidance on remote inspections ("Remote Interactive Evaluations") in light of COVID-19, suggesting that the FDA may continue to use remote inspections to supplement on-site audits post-pandemic. The European Federation of Pharmaceutical Industries and Associations (EFPIA) also predicts added value in retaining a virtual inspection system beyond the pandemic.
By using all available approaches to ensure drug products are safe, effective, and of high quality, regulatory agencies agree that this form of evaluation is an effective and adaptive strategy for the duration of the COVID-19 public health emergency. James Choi, Samsung Biologics Senior VP and Chief Information and Marketing Officer said, "Our Live Virtual Tour platform not only fully complies with the regulatory agencies’ guidance on remote interactive evaluations but is also aimed at promoting greater client satisfaction through digital and virtual access to our site and quality documents."
About Samsung Biologics Co., Ltd.
Samsung Biologics (KRX: 207940.KS) is a fully integrated CDMO offering state-of-the-art contract development, manufacturing, and laboratory testing services. With proven regulatory approvals, the largest capacity, and the fastest throughput, Samsung Biologics is an award-winning partner of choice and is uniquely able to support the development and manufacturing of biologics products at every stage of the process while meeting the evolving needs of biopharmaceutical companies worldwide. For more information, visit www.samsungbiologics.com.
Samsung Biologics
Media Contact:
Claire Kim
Senior Director, Global Public Relations
[email protected]
Related Links :
https://samsungbiologics.com/en